Tongze Hexin (Beijing) pharmaceutical technology has always adhered to the "credit and service first" and made great efforts to provide efficient and high-quality professional services for customers at home and abroad.
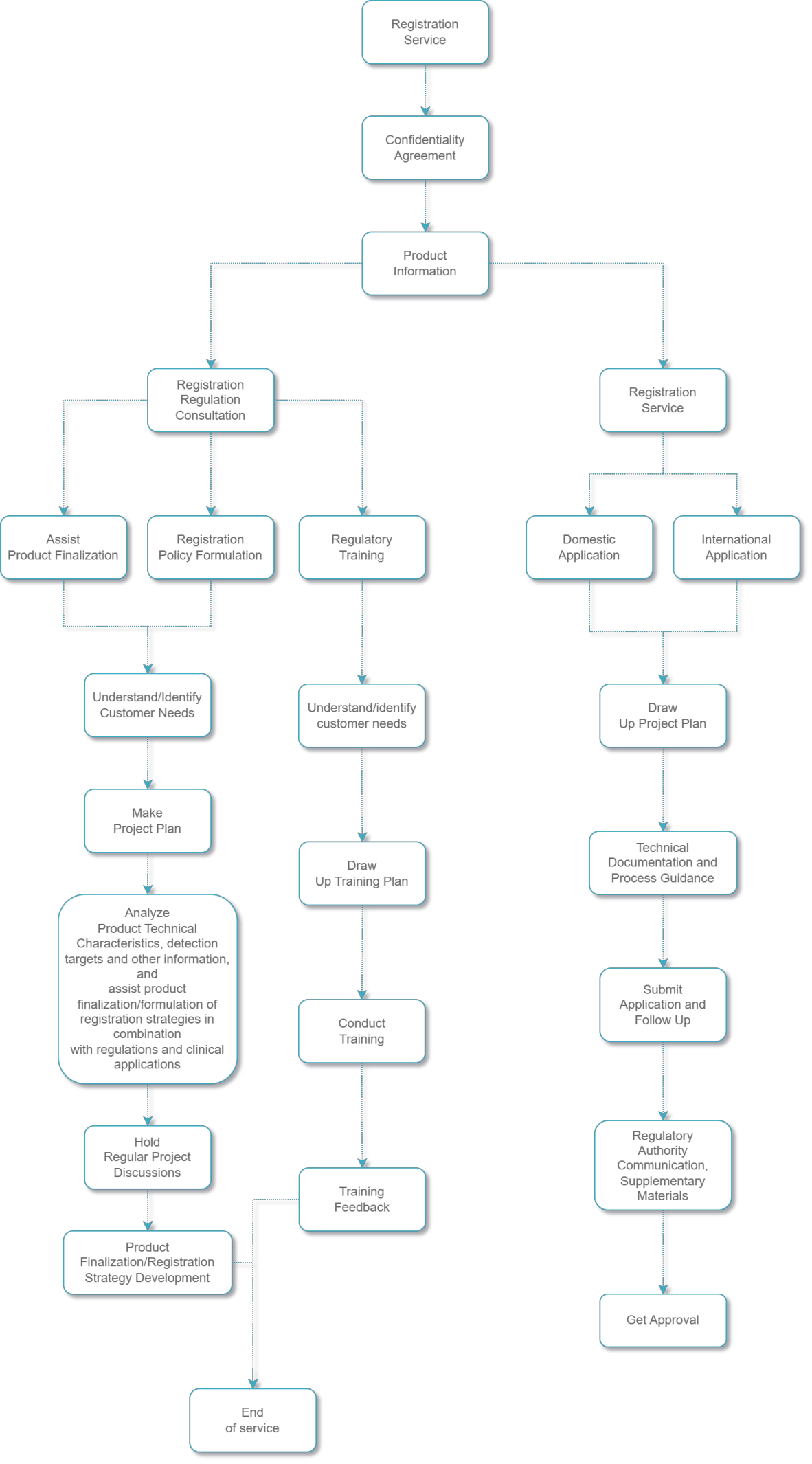
Providing registration and application consulting services for domestic and foreign enterprises engaged in the development of IVD products (reagents, instruments, software), and are committed to providing complete solutions in various key segments such as regulatory policy consultation, registration strategy planning, product research and development, registration testing/self testing, registration application, and help products obtaining NMPA, FDA, CE, and WHO approval for market listing.
|
Registration strategic planning |
Registration documentation writing consultation |
Registration and declaration service |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Registration department personnel all have been engaged in registration positions in diagnostic reagents and medical device related enterprises, and have rich experience in the registration and declaration of in vitro diagnostic reagents, instruments and software
Carefully interpret the registration laws and regulations for customers, formulate custom made special registration strategy and data requirements for each product, and assist customers to achieve faster and better product development.
Assist customers to carry out registration testing and registration application quickly and efficiently, avoid risk points to avoid repeated operations, saving time and cost.